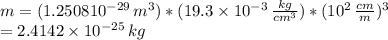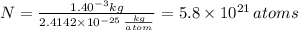Chemistry, 23.09.2019 05:30 robloxlover1987
The atomic radius of a gold atom is 144×10^-12. the volume of a gold atom can be calculated using the volume of a sphere. the density of gold is 19.3 g/cm^3. how many atoms are present in a sample of gold with a mass of 1.40g using the info provided

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

Chemistry, 23.06.2019 00:10
Apropane torch is lit inside a hot air balloon during preflight preparations to inflate the balloon. which condition of the gas remains constant
Answers: 2
You know the right answer?
The atomic radius of a gold atom is 144×10^-12. the volume of a gold atom can be calculated using th...
Questions

Mathematics, 11.12.2021 01:30






Mathematics, 11.12.2021 01:30


Mathematics, 11.12.2021 01:30


Arts, 11.12.2021 01:30


Mathematics, 11.12.2021 01:30


Mathematics, 11.12.2021 01:30




German, 11.12.2021 01:30

Chemistry, 11.12.2021 01:30