
Chemistry, 23.09.2019 14:20 demetriascott20
Iron ore is reduced to pure iron by smelting, during which the iron (iii) oxide in the ore reacts with carbon monoxide gas, like this: fe2o3s + 3cog → 2fes + 3co2g suppose an engineer decides to study the rate of this reaction. she prepares four reaction vessels with 158.8g of solid iron (iii) oxide and 33.9g of carbon monoxide gas each. the volume and temperature of each vessel is shown in the table below. arrange the reaction vessels in decreasing order of initial rate of reaction. in other words, select a "1" next to the vessel in which the engineer can reasonably expect the initial rate of reaction to be highest, a "2" next to the vessel in which the initial rate of reaction would be next highest, and so on. rate of reaction a 2.0l 1100.°c b 2.0l 1200.°c c 4.0l 1100.°c d 4.0l 1000.°c

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3

Chemistry, 23.06.2019 00:00
This statement about matter and its behavior is best classified as a
Answers: 1
You know the right answer?
Iron ore is reduced to pure iron by smelting, during which the iron (iii) oxide in the ore reacts wi...
Questions


Mathematics, 01.02.2020 23:45



Mathematics, 01.02.2020 23:45



English, 01.02.2020 23:45

Mathematics, 01.02.2020 23:45

History, 01.02.2020 23:45

Mathematics, 01.02.2020 23:45


Biology, 01.02.2020 23:45

English, 01.02.2020 23:45

Mathematics, 01.02.2020 23:45

World Languages, 01.02.2020 23:45


History, 01.02.2020 23:45

Mathematics, 01.02.2020 23:45

History, 01.02.2020 23:45

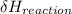 = negative.
= negative.

