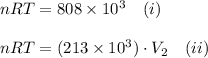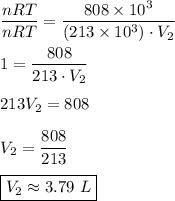Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1


Chemistry, 23.06.2019 05:00
How is electrolysis most commonly used to produce an energy source? a - splitting water molecules produces oxygen, which organisms breathe to fuel their bodies. b - splitting water molecules produces hydrogen gas, which is used to power machines through hydrogen fuel cells. c - splitting carbon dioxide molecules produces coal, a form of carbon that can be burned to produce heat. d - splitting carbon dioxide molecules produces natural gas, which can be burned to generate electricity in power plants.
Answers: 1
You know the right answer?
The gas in a cylinder has a volume of 8 liters at a pressure of 101 kpa. the pressure of the gas is...
Questions


English, 21.10.2021 01:00

SAT, 21.10.2021 01:00

Mathematics, 21.10.2021 01:00


History, 21.10.2021 01:00




Mathematics, 21.10.2021 01:00


History, 21.10.2021 01:00

Biology, 21.10.2021 01:00

Mathematics, 21.10.2021 01:00

Health, 21.10.2021 01:00

Mathematics, 21.10.2021 01:00

Mathematics, 21.10.2021 01:00

Mathematics, 21.10.2021 01:00

Mathematics, 21.10.2021 01:00

Mathematics, 21.10.2021 01:00

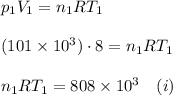
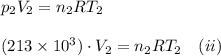
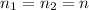 . Furthermore, the temperature remains constant, so
. Furthermore, the temperature remains constant, so 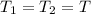 . Then, the expressions that were find are:
. Then, the expressions that were find are: