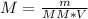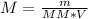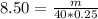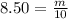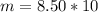Chemistry, 18.09.2019 00:30 moongirl4791
You have been asked to prepare an 8.50m solution of sodium hydroxide, naoh in a 250ml volumetric flask. how many grams of solute will you have to mass on the balance?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 13:30
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1

Chemistry, 22.06.2019 18:00
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1
You know the right answer?
You have been asked to prepare an 8.50m solution of sodium hydroxide, naoh in a 250ml volumetric fla...
Questions

Advanced Placement (AP), 02.11.2020 20:10

Mathematics, 02.11.2020 20:10

History, 02.11.2020 20:10




Chemistry, 02.11.2020 20:10


Mathematics, 02.11.2020 20:10


Mathematics, 02.11.2020 20:10

Mathematics, 02.11.2020 20:10

Physics, 02.11.2020 20:10


Mathematics, 02.11.2020 20:10




Arts, 02.11.2020 20:10

Social Studies, 02.11.2020 20:10