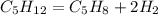How many milliliters of c5h12 are needed to make 97.3 ml c5h8?
density of c5h12 = 0.620 g/ml<...

Chemistry, 30.08.2019 12:00 naimareiad
How many milliliters of c5h12 are needed to make 97.3 ml c5h8?
density of c5h12 = 0.620 g/ml
density of c5h8 = 0.681 g/ml
density of h2= 0.0899g/l
explain

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 16:00
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2
You know the right answer?
Questions



Mathematics, 16.05.2021 22:50

Social Studies, 16.05.2021 22:50

Mathematics, 16.05.2021 22:50

Mathematics, 16.05.2021 22:50



Mathematics, 16.05.2021 22:50

Mathematics, 16.05.2021 22:50

Mathematics, 16.05.2021 22:50


History, 16.05.2021 22:50


History, 16.05.2021 22:50

Biology, 16.05.2021 22:50



Mathematics, 16.05.2021 22:50