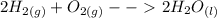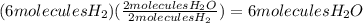Chemistry, 06.10.2019 15:00 Averyruss9245
How many water molecules (h2o) can be produced from 6 molecules of hydrogen gas (purple) reacting with 6 molecules of oxygen gas (turquoise)? which reactant is the limiting reactant?
oxygen and hydrogen molecules
a. 6 water molecules, hydrogen is limiting
b. 3 water molecules, oxygen is limiting
c. 3 water molecules, hydrogen is limiting
d. 12 water molecules, oxygen is limiting

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1
You know the right answer?
How many water molecules (h2o) can be produced from 6 molecules of hydrogen gas (purple) reacting wi...
Questions


Mathematics, 30.05.2020 01:03

Mathematics, 30.05.2020 01:03

Mathematics, 30.05.2020 01:03


History, 30.05.2020 01:03

Biology, 30.05.2020 01:03

Mathematics, 30.05.2020 01:03

Physics, 30.05.2020 01:03

History, 30.05.2020 01:03

Chemistry, 30.05.2020 01:03



Mathematics, 30.05.2020 01:03

English, 30.05.2020 01:03