
Chemistry, 31.08.2019 13:00 coolkiddKC
Ga sealed container holds 0.020 moles of nitrogen (n2) gas, at a pressure of 1.5 atmospheres and a temperature of 290 k. the atomic mass of nitrogen is 14.0 g/mol. the boltzmann constant is 1.38 × 10-23 j/k and the ideal gas constant is r = 8.314 j/ mol · k = 0.0821 l · atm/mol · k. the mass density of the gas is closest to

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1


Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 23.06.2019 00:30
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1
You know the right answer?
Ga sealed container holds 0.020 moles of nitrogen (n2) gas, at a pressure of 1.5 atmospheres and a t...
Questions

Mathematics, 06.05.2021 22:10

Mathematics, 06.05.2021 22:10



Arts, 06.05.2021 22:10



Mathematics, 06.05.2021 22:10



Mathematics, 06.05.2021 22:10

Mathematics, 06.05.2021 22:10


Biology, 06.05.2021 22:10

Mathematics, 06.05.2021 22:10

Mathematics, 06.05.2021 22:10


Mathematics, 06.05.2021 22:10


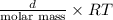
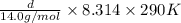
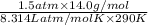
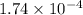 g
g

