
One way the u. s. environmental protection agency (epa) tests for chloride contaminants in water is by titrating a sample of silver nitrate solution. any chloride anions in solution will combine with the silver cations to produce bright white silver chloride precipitate. suppose an epa chemist tests a 200.ml sample of groundwater known to be contaminated with copper(ii) chloride, which would react with silver nitrate solution like this: cucl2 (aq) + 2agno3 (aq) → 2agcl (s) + cuno32 (aq) the chemist adds 26.0m m silver nitrate solution to the sample until silver chloride stops forming. she then washes, dries, and weighs the precipitate. she finds she has collected 6.1mg of silver chloride. calculate the concentration of copper(ii) chloride contaminant in the original groundwater sample. be sure your answer has the correct number of significant digits.

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 06:30
The minerals found in bones are deposited by living cells called
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3
You know the right answer?
One way the u. s. environmental protection agency (epa) tests for chloride contaminants in water is...
Questions

Mathematics, 01.04.2021 06:20

Computers and Technology, 01.04.2021 06:20

Mathematics, 01.04.2021 06:20

Mathematics, 01.04.2021 06:20

Mathematics, 01.04.2021 06:20



Mathematics, 01.04.2021 06:20

Mathematics, 01.04.2021 06:20


Mathematics, 01.04.2021 06:20


History, 01.04.2021 06:20

Chemistry, 01.04.2021 06:20




Biology, 01.04.2021 06:20

English, 01.04.2021 06:20

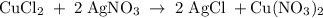
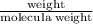
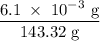
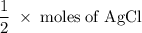
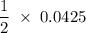 mmoles
mmoles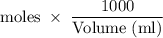
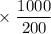 mM
mM

