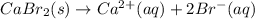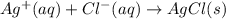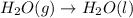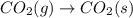Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

Chemistry, 22.06.2019 23:00
In the reaction h2co3 (aq) + 3nh3 (aq) = 2 nh4+ (aq) + co3 2-, how many electrons are transferred?
Answers: 3

You know the right answer?
For which of the following processes would you expect there to be an increase in entropy? ag+(aq) +...
Questions




History, 29.04.2021 19:40

English, 29.04.2021 19:40

Physics, 29.04.2021 19:40

Mathematics, 29.04.2021 19:40


Mathematics, 29.04.2021 19:40

Mathematics, 29.04.2021 19:40


Social Studies, 29.04.2021 19:40

Social Studies, 29.04.2021 19:40


Chemistry, 29.04.2021 19:40

Mathematics, 29.04.2021 19:40


History, 29.04.2021 19:40