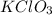Chemistry, 23.09.2019 11:30 nauticatyson9
A13.00 g sample of a compound contains 4.15 g potassium (k), 3.76 g chlorine (cl), and oxygen (o). calculate the empirical formula.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:40
If 10.0 ml of the solution on the right are withdrawn from the 100 ml beaker and diluted again in a similar manner, what is the new concentration? m nacl
Answers: 2

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 07:00
Which set of characteristics best describes igneous rock? a) largest type of rock, made of organic matter, hardest type of rock b) least abundant type of rock, made of other rocks, made mostly of minerals c) found on all continents, contains wavy bands of stripes, contains fossils d) most abundant type in earth's crust, made of magma/lava, contains no fossils
Answers: 1

You know the right answer?
A13.00 g sample of a compound contains 4.15 g potassium (k), 3.76 g chlorine (cl), and oxygen (o). c...
Questions

History, 21.04.2020 04:52

English, 21.04.2020 04:52

Mathematics, 21.04.2020 04:52




History, 21.04.2020 04:52

Mathematics, 21.04.2020 04:52


Mathematics, 21.04.2020 04:52

Arts, 21.04.2020 04:52






Chemistry, 21.04.2020 04:52