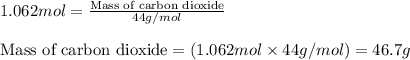Chemistry, 09.10.2019 01:30 joshua1255
What mass of carbon dioxide (co2) can be produced from 15.6 g of c6h14 and excess oxygen?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3


Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2
You know the right answer?
What mass of carbon dioxide (co2) can be produced from 15.6 g of c6h14 and excess oxygen?...
Questions

Mathematics, 13.02.2021 06:20


History, 13.02.2021 06:20

Mathematics, 13.02.2021 06:20

Mathematics, 13.02.2021 06:20

Physics, 13.02.2021 06:20

English, 13.02.2021 06:20


Mathematics, 13.02.2021 06:20

English, 13.02.2021 06:20




Mathematics, 13.02.2021 06:20

Mathematics, 13.02.2021 06:20

Chemistry, 13.02.2021 06:20


Arts, 13.02.2021 06:20

Geography, 13.02.2021 06:20

Mathematics, 13.02.2021 06:20

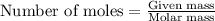 .....(1)
.....(1)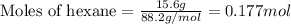
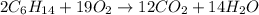
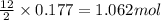 of carbon dioxide
of carbon dioxide