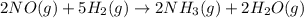Chemistry, 07.12.2019 16:31 andygomez1312M
The balanced chemical equation, 2no (g) + 5h2 (g) (arrow pointing this way > > ) 2nh3 (g) + 2h2o (g), can be expressed in words as:
nitrogen dioxide gas plus hydrogen gas yields ammonia gas plus water
nitrogen monoxide gas plus hydrogen gas yields ammonia gas plus water vapor
nickel monoxide gas plus hydrogen gas yields nitrogen trihydride plus water
nitrogen oxide gas plus hydrogen gas yields ammonia gas plus water vapor
i think it's either b, or d. i can't figure out which one it is though. .

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1

Chemistry, 23.06.2019 07:30
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes no correct anwser get brainliest
Answers: 1

Chemistry, 23.06.2019 08:00
The goal of this experiment was to answer the question "what is the effect of a gas' temperature on its volume? " you formulated the hypothesis below. hypothesis: if a fixed amount of gas is heated, then the volume will increase because the heat will cause the molecules of gas to move faster and further apart. to test this hypothesis, you changed the of the gas between 0 and 100°c (273 and 373 k) and calculated the resulting of the gas.
Answers: 2
You know the right answer?
The balanced chemical equation, 2no (g) + 5h2 (g) (arrow pointing this way > > ) 2nh3 (g) + 2h...
Questions

History, 02.07.2019 18:00

Social Studies, 02.07.2019 18:00

History, 02.07.2019 18:00

Biology, 02.07.2019 18:00

Social Studies, 02.07.2019 18:00


History, 02.07.2019 18:00

History, 02.07.2019 18:00

English, 02.07.2019 18:00

Mathematics, 02.07.2019 18:00



English, 02.07.2019 18:00

Mathematics, 02.07.2019 18:00

Mathematics, 02.07.2019 18:00


History, 02.07.2019 18:00

Health, 02.07.2019 18:00


English, 02.07.2019 18:00