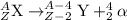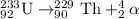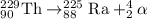Chemistry, 23.09.2019 15:00 madisonreynolds2208
Uranium-233 undergoes alpha decay, and then the daughter isotope undergoes another alpha decay. which equation correctly describes this decay series? use the periodic table link from the tools bar to answer the question.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Alculate the concentration of h3o⁺in a solution that contains 5.5 × 10-5m oh⁻at 25°c. identify the solution as acidic, basic, or neutral.a) 1.8 × 10-10m, basicb) 1.8 × 10-10m, acidicc) 5.5 × 10-10m, neutrald) 9.2 × 10-1m, acidice) 9.2 × 10-1m, basic
Answers: 1

Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1
You know the right answer?
Uranium-233 undergoes alpha decay, and then the daughter isotope undergoes another alpha decay. whic...
Questions


Mathematics, 26.06.2019 02:40






English, 26.06.2019 02:40

Mathematics, 26.06.2019 02:40





Chemistry, 26.06.2019 02:40





Mathematics, 26.06.2019 02:40