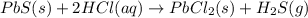Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Which of the following best describes the formation of plasma?
Answers: 1

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3
You know the right answer?
Solid lead(ii) sulfide reacts with aqueous hydrochloric acid to form solid lead(ii) chloride and dih...
Questions

Social Studies, 08.03.2021 22:40

Biology, 08.03.2021 22:40

Geography, 08.03.2021 22:40

Social Studies, 08.03.2021 22:40

Mathematics, 08.03.2021 22:40

Mathematics, 08.03.2021 22:40

Chemistry, 08.03.2021 22:40

Mathematics, 08.03.2021 22:40



Physics, 08.03.2021 22:40

Mathematics, 08.03.2021 22:40

History, 08.03.2021 22:40

Mathematics, 08.03.2021 22:40


Mathematics, 08.03.2021 22:40



English, 08.03.2021 22:40