

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

Chemistry, 23.06.2019 00:10
Find the missing probability in the table below a.0.10 b.40 c.0.80 d. 0.20
Answers: 2

Chemistry, 23.06.2019 11:00
Intermolecular forces. question i need with: the only intermolecular forces that affect non polar molecules are forces.
Answers: 2
You know the right answer?
Manganese dioxide (mno2(s), hf = –520.0 kj) reacts with aluminum to form aluminum oxide (ai2o3(s), h...
Questions

Chemistry, 09.03.2021 09:30


History, 09.03.2021 09:30



Social Studies, 09.03.2021 09:30

History, 09.03.2021 09:30


English, 09.03.2021 09:30

History, 09.03.2021 09:30

History, 09.03.2021 09:30

Mathematics, 09.03.2021 09:30

Mathematics, 09.03.2021 09:30

Chemistry, 09.03.2021 09:30

Mathematics, 09.03.2021 09:30

Social Studies, 09.03.2021 09:30

Chemistry, 09.03.2021 09:30



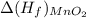 = -520.0 KJ/mole
= -520.0 KJ/mole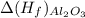 = -1699.8 KJ/mole
= -1699.8 KJ/mole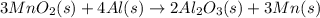
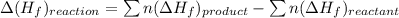
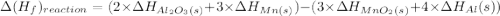
![\Delta (H_{f})_{reaction}=[2moles\times (-1699.8 KJ/mole)}+3moles\times (0\text{ KJ/mole}})]-[(3moles\times(-520.0KJ/mole }+4moles\times(0\text{ KJ/mole})]](/tpl/images/0218/2536/8178d.png)


