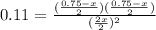Chemistry, 29.09.2019 15:30 rebecca7415
At a certain temperature the equilibrium constant, kc, equals 0.11 for the reaction: 2 icl(g)? i2(g) + cl2(g). what is the equilibrium concentration of icl if 0.75 mol of i2 and 0.75 mol of cl2 are initially mixed in a 2.0-l flask?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Infants born with severe respiratory problems are sometimes given liquid ventilation: they breathe a liquid that can dissolve more oxygen than air can hold. one of these liquids is a fluorinated compound, cf3(cf2)7br. the solubility of oxygen in this liquid is 66 mlo2 per 100 ml liquid. in contrast, air is 21 % oxygen by volume. calculate the moles of o2 present in an infant's lungs (volume: 12 ml ) if the infant takes a full breath of air. assume a pressure of 1 atm in the lungs.
Answers: 1

Chemistry, 21.06.2019 13:00
What type of reaction is represented by the following example? 2co2 (g) + 4h2o (l) + 1452 kj 2ch3oh (l) (g) + 3o2 (g) exothermic endothermic
Answers: 1

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1
You know the right answer?
At a certain temperature the equilibrium constant, kc, equals 0.11 for the reaction: 2 icl(g)? i2(g...
Questions




Mathematics, 12.03.2021 02:40

Mathematics, 12.03.2021 02:40

Mathematics, 12.03.2021 02:40

Mathematics, 12.03.2021 02:40




Mathematics, 12.03.2021 02:50

Mathematics, 12.03.2021 02:50

Mathematics, 12.03.2021 02:50



Health, 12.03.2021 02:50



Mathematics, 12.03.2021 02:50

Mathematics, 12.03.2021 02:50