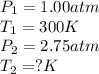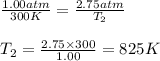Aflask of fixed volume contains 1.00 mole of gaseous carbon dioxide and 88.0 g of solid carbon dioxide. the original pressure and temperature in the flask is 1.00 atm and 300. k. all of the solid carbon dioxide sublimes. the final pressure in the flask is 2.75 atm. what is the final temperature in kelvins? assume the solid carbon dioxide takes up negligible volume.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3

Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

Chemistry, 22.06.2019 23:00
What prefix multiplier is appropriate for reporting a measurement of 5.57 ×10−5 m?
Answers: 1
You know the right answer?
Aflask of fixed volume contains 1.00 mole of gaseous carbon dioxide and 88.0 g of solid carbon dioxi...
Questions









Computers and Technology, 10.09.2019 18:30











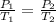 (at constant volume)
(at constant volume)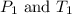 are the initial pressure and temperature of the gas.
are the initial pressure and temperature of the gas.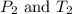 are the final pressure and temperature of the gas.
are the final pressure and temperature of the gas.