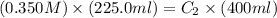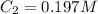Chemistry, 24.12.2019 19:31 odymonster9
Initially a beaker contains 225.0 ml of a 0.350 m mgso4 solution. then 175.0 ml of water are added to the beaker. find the concentration of the final solution. what is the volume?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Read the given expression. x = number of protons − number of core electrons which of the following explains the identity of x and its trends across a period? x is the effective nuclear charge, and it remains constant across a period. x is the screening constant, and it remains constant across a period. x is the effective nuclear charge, and it increases across a period. x is the screening constant, and it increases across a period.
Answers: 1

Chemistry, 21.06.2019 17:30
One mole of zinc has a mass of 65.4 grams. approximately how many atoms of zinc are present in one mole of zinc? 32 × 1023 atoms 6 × 1023 atoms 66 atoms 65 atoms
Answers: 1

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1
You know the right answer?
Initially a beaker contains 225.0 ml of a 0.350 m mgso4 solution. then 175.0 ml of water are added t...
Questions



Computers and Technology, 27.09.2019 23:30

English, 27.09.2019 23:30


Computers and Technology, 27.09.2019 23:30

Social Studies, 27.09.2019 23:30





Mathematics, 27.09.2019 23:30

Mathematics, 27.09.2019 23:30



English, 27.09.2019 23:30

Mathematics, 27.09.2019 23:30


Mathematics, 27.09.2019 23:30

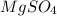 + Volume of water added
+ Volume of water added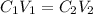
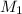 = concentration of
= concentration of 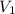 = volume of
= volume of 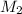 = concentration of final solution = ?
= concentration of final solution = ?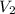 = volume of final solution = 400 ml
= volume of final solution = 400 ml