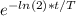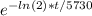Chemistry, 09.10.2019 17:00 angellight4all
Avery old tree limb contains an amount of carbon-14 that is approximately 1/8 of the current atmospheric 14c levels.. calculate your answers to the following and be sure to show all steps of your calculations.
• approximately how long ago was the tree cut down?
• if there were 1,500,000 carbon-14 atoms to begin with, how many atoms would remain

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1
You know the right answer?
Avery old tree limb contains an amount of carbon-14 that is approximately 1/8 of the current atmosph...
Questions

Social Studies, 20.08.2019 03:50

Mathematics, 20.08.2019 03:50

Health, 20.08.2019 03:50


Mathematics, 20.08.2019 03:50

English, 20.08.2019 03:50

Biology, 20.08.2019 03:50

Mathematics, 20.08.2019 03:50

History, 20.08.2019 03:50

Computers and Technology, 20.08.2019 03:50

Mathematics, 20.08.2019 03:50

Chemistry, 20.08.2019 03:50

Biology, 20.08.2019 03:50

Mathematics, 20.08.2019 03:50

Mathematics, 20.08.2019 03:50

English, 20.08.2019 03:50


Mathematics, 20.08.2019 03:50


History, 20.08.2019 03:50