What is oxidized and what is reduced in this reaction?
cu(s) + 2agno3(aq) → cu(no3)2(aq...

Chemistry, 24.01.2020 13:31 barkahigh8089
What is oxidized and what is reduced in this reaction?
cu(s) + 2agno3(aq) → cu(no3)2(aq) + 2ag(s)
cu is reduced and ag is oxidized.
ag is reduced and cu is oxidized.
no3 is oxidized and cu is reduced.
ag is oxidized and no3 is reduced.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
In pea plants, the allele for tallness (t) is dominant to the allele for shortness (t). in the cross between a tall pea plant and a short pea plant shown below, what is the probability that the resulting offspring will be tall? whats the percent
Answers: 1


Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
You know the right answer?
Questions

Chemistry, 19.04.2021 15:30

Mathematics, 19.04.2021 15:30








Mathematics, 19.04.2021 15:30










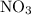 , and Ag in the reactants is 0, -1, and +1 respectively.
, and Ag in the reactants is 0, -1, and +1 respectively.

