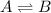Chemistry, 28.01.2020 12:31 awsomeboy12345678
Two gases are placed in a sealed flask and allowed to react. which statement is true about this closed system when it reaches dynamic equilibrium? both reactants and products will be present, but forward and reverse reactions will stop. particles of the products will be present, but no particles of the reactants will remain. the concentration of the reactants and the concentration of the products will be equal. the rate of the forward reaction and the rate of the reverse reaction will be equal.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1



Chemistry, 22.06.2019 23:00
Which type of intermolecular attractions holds ammonia molecules together with other ammonia molecules?
Answers: 3
You know the right answer?
Two gases are placed in a sealed flask and allowed to react. which statement is true about this clos...
Questions

Spanish, 10.03.2021 09:20

English, 10.03.2021 09:20

Spanish, 10.03.2021 09:20


Mathematics, 10.03.2021 09:20

Computers and Technology, 10.03.2021 09:20



Mathematics, 10.03.2021 09:20

Mathematics, 10.03.2021 09:20



Mathematics, 10.03.2021 09:20

History, 10.03.2021 09:20


Mathematics, 10.03.2021 09:20

Mathematics, 10.03.2021 09:20

Mathematics, 10.03.2021 09:20

Mathematics, 10.03.2021 09:20

Geography, 10.03.2021 09:20