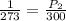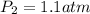Neon gas occupies a metal container of 5.5 l at stp (273 k, 1 atm). the volume of the metal container cannot be changed. what will happen to the pressure if the temperature rises to 300k? a. it will fall to 0.7 atm. b. it will fall to 0.9 atm. c. it will rise to 1.1 atm. d. it will rise to 1.3 atm.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Energy is released during which phase changes? check all that apply. boiling condensing depositing freezing melting subliming
Answers: 2

Chemistry, 22.06.2019 01:30
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3
You know the right answer?
Neon gas occupies a metal container of 5.5 l at stp (273 k, 1 atm). the volume of the metal containe...
Questions

Mathematics, 03.02.2020 22:47


English, 03.02.2020 22:47

Chemistry, 03.02.2020 22:47

Business, 03.02.2020 22:47





Biology, 03.02.2020 22:47

Mathematics, 03.02.2020 22:47

Mathematics, 03.02.2020 22:47

Mathematics, 03.02.2020 22:47

Mathematics, 03.02.2020 22:47

Biology, 03.02.2020 22:47

English, 03.02.2020 22:47

Mathematics, 03.02.2020 22:47



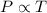 (At constant volume and number of moles)
(At constant volume and number of moles)
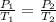
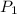 = initial pressure of gas = 1 atm
= initial pressure of gas = 1 atm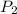 = final pressure of gas = ?
= final pressure of gas = ?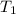 = initial temperature of gas =
= initial temperature of gas = 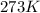
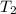 = final temperature of gas = 300 K
= final temperature of gas = 300 K