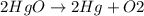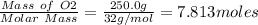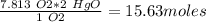Chemistry, 24.09.2019 11:30 vickygloom
Mercury(ii) oxide (hgo) decomposes to form mercury (hg) and oxygen (o2). the balanced chemical equation is shown below. 2hgo mc020-1.jpg 2hg + o2 the molar mass of hgo is 216.59 g/mol. the molar mass of o2 is 32.00 g/mol. how many moles of hgo are needed to produce 250.0 g of o2?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2
You know the right answer?
Mercury(ii) oxide (hgo) decomposes to form mercury (hg) and oxygen (o2). the balanced chemical equat...
Questions

Mathematics, 08.12.2020 07:40


English, 08.12.2020 07:40

Physics, 08.12.2020 07:40


Mathematics, 08.12.2020 07:40

English, 08.12.2020 07:40

English, 08.12.2020 07:40





Mathematics, 08.12.2020 07:40




Chemistry, 08.12.2020 07:40


Mathematics, 08.12.2020 07:40