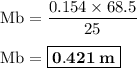Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Diffraction is when light is bent around obstructions. which of the these observation about clouds would indicate diffraction? a) after rain storms, you can sometimes see rainbows. b) clouds are white or gray and cannot be seen through. c) on a cloudy day, the temperature tends to be cooler than a sunny day. d) the edges of dark clouds appear lighter. this
Answers: 3

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 23.06.2019 04:31
How does a sample of helium at 15 degree celsius compare to a sample of helium at 215 k? a) the helium at 15 degrees celsius has a higher average kinetic energy that the sample at 215 k. b) the helium at 15 degrees celsius has lower nuclear energy that the sample at 215 k. c) the helium at 15 degrees celsius has slower- moving atoms that the sample at 215 k. d) the helium at 15 degrees celsius has smaller atoms than the sample at 215 k.
Answers: 1
You know the right answer?
By titration it is found that 68.5 ml of 0.154 m naoh(aq) is needed to neutralize 25.0 ml of hcl(aq)...
Questions

Mathematics, 19.08.2019 20:30



Chemistry, 19.08.2019 20:30


Chemistry, 19.08.2019 20:30

History, 19.08.2019 20:30

Physics, 19.08.2019 20:30





Social Studies, 19.08.2019 20:30

English, 19.08.2019 20:30

History, 19.08.2019 20:30

Biology, 19.08.2019 20:30

Social Studies, 19.08.2019 20:30

History, 19.08.2019 20:30

Biology, 19.08.2019 20:30

Social Studies, 19.08.2019 20:30