

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 04:00
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1
You know the right answer?
In a disproportionation reaction, the disproportionate substance
has exactly one oxidation st...
has exactly one oxidation st...
Questions

Biology, 21.10.2021 14:00

English, 21.10.2021 14:00

Health, 21.10.2021 14:00

Computers and Technology, 21.10.2021 14:00


English, 21.10.2021 14:00

Mathematics, 21.10.2021 14:00

History, 21.10.2021 14:00

History, 21.10.2021 14:00



Chemistry, 21.10.2021 14:00

Mathematics, 21.10.2021 14:00

Law, 21.10.2021 14:00

Physics, 21.10.2021 14:00

Mathematics, 21.10.2021 14:00

Biology, 21.10.2021 14:00

Chemistry, 21.10.2021 14:00

Geography, 21.10.2021 14:00

Mathematics, 21.10.2021 14:00

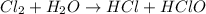
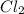 to HCl. The oxidation state of chlorine is reducing from 0 to -1.
to HCl. The oxidation state of chlorine is reducing from 0 to -1.

