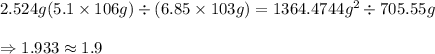Chemistry, 02.10.2019 14:10 PaMuth1100
2.524 g (5.1 × 106 g) ÷ (6.85 × 103 g) = ? how many significant figures should the result have?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Acycloalkane molecule contains 8 carbon atoms. how many hydrogen atoms are present in the molecule?
Answers: 2

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 22.06.2019 19:00
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1
You know the right answer?
2.524 g (5.1 × 106 g) ÷ (6.85 × 103 g) = ? how many significant figures should the result have?...
Questions




Biology, 25.10.2021 14:00

Mathematics, 25.10.2021 14:00

Physics, 25.10.2021 14:00


Mathematics, 25.10.2021 14:00


Mathematics, 25.10.2021 14:00


Computers and Technology, 25.10.2021 14:00


Mathematics, 25.10.2021 14:00

Mathematics, 25.10.2021 14:00


Mathematics, 25.10.2021 14:00

Spanish, 25.10.2021 14:00

Mathematics, 25.10.2021 14:00