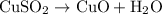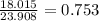Chemistry, 16.09.2019 18:40 juicemankinnie95
In the decomposition reaction, 1 mole of water (mw = 18.015 g/mol) was produced for every mole of cuo (mw = 79.545 g/mol) produced. given that 3.327 g of cuo was produced during the reaction, how many grams of water were released as water vapor? (number of moles = mass (g) / molar mass (g/ choose the closest answer.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:10
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 17:10
In which block of the periodic table is uranium (u) found? s blockd blockp blockf block
Answers: 1


Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer
Answers: 1
You know the right answer?
In the decomposition reaction, 1 mole of water (mw = 18.015 g/mol) was produced for every mole of cu...
Questions

Geography, 21.04.2020 22:37

Mathematics, 21.04.2020 22:37

Biology, 21.04.2020 22:37

Mathematics, 21.04.2020 22:37




Mathematics, 21.04.2020 22:37

Mathematics, 21.04.2020 22:37

Computers and Technology, 21.04.2020 22:37








Computers and Technology, 21.04.2020 22:37