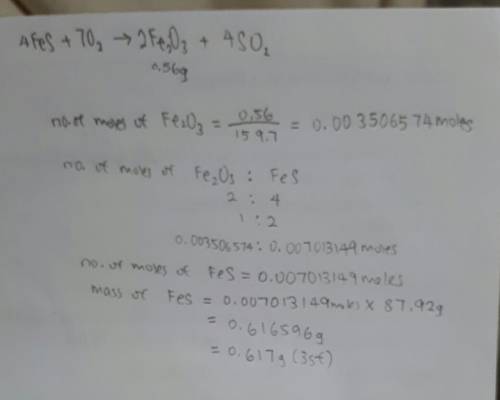In the following reaction, how many grams of ferrous sulfide (fes) will produce
0.56 grams of...


Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3

Chemistry, 23.06.2019 01:30
What happens to the concentration of hydronium ions as the ph of a solution increases? a. hydronium ion concentration stays the same b. hydronium ion concentration decreases c. hydronium ion concentration increases
Answers: 1
You know the right answer?
Questions

Mathematics, 08.10.2019 22:30


English, 08.10.2019 22:30

History, 08.10.2019 22:30


Mathematics, 08.10.2019 22:30

Social Studies, 08.10.2019 22:30

Mathematics, 08.10.2019 22:30


Mathematics, 08.10.2019 22:30

Biology, 08.10.2019 22:30

Physics, 08.10.2019 22:30






Biology, 08.10.2019 22:30

Mathematics, 08.10.2019 22:30