

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

Chemistry, 22.06.2019 14:00
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3
You know the right answer?
Consider the reaction below. 2al2o3 4al + 3o2 how many moles of oxygen are produced when 26.5 mol of...
Questions

Mathematics, 22.10.2020 14:01

Social Studies, 22.10.2020 14:01

History, 22.10.2020 14:01


Mathematics, 22.10.2020 14:01

Advanced Placement (AP), 22.10.2020 14:01

Mathematics, 22.10.2020 14:01


Mathematics, 22.10.2020 14:01

Mathematics, 22.10.2020 14:01


Mathematics, 22.10.2020 14:01




World Languages, 22.10.2020 14:01




Physics, 22.10.2020 14:01

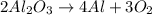
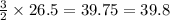 moles of oxygen
moles of oxygen

