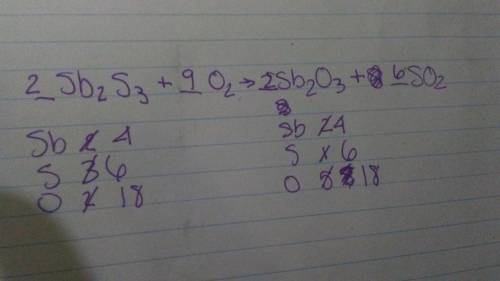Chemistry, 09.01.2020 09:31 lucerogon7403
Complete the balancing of the equation for the roasting of stibnite, using smallest whole-number coefficients. sb2s3+o2 yields sb2o3+so2. explain because i don't understand this specific question

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
You know the right answer?
Complete the balancing of the equation for the roasting of stibnite, using smallest whole-number coe...
Questions


English, 21.04.2021 16:10


Mathematics, 21.04.2021 16:10




Chemistry, 21.04.2021 16:10

World Languages, 21.04.2021 16:10

Mathematics, 21.04.2021 16:10

Mathematics, 21.04.2021 16:10




Mathematics, 21.04.2021 16:10




English, 21.04.2021 16:10

Mathematics, 21.04.2021 16:10