
Chemistry, 02.09.2019 19:00 deanlmartin
A50.00 g sample of an unknown metal is heated to 45.00°c. it is then placed in a coffee-cup calorimeter filled with water. the calorimeter and the water have a combined mass of 250.0 g and an overall specific heat of 1.035 cal/g•°c. the initial temperature of the calorimeter is 10.00°c. the system reaches a final temperature of 11.08°c when the metal is added.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1

Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1
You know the right answer?
A50.00 g sample of an unknown metal is heated to 45.00°c. it is then placed in a coffee-cup calorime...
Questions


Mathematics, 26.06.2019 13:50



Mathematics, 26.06.2019 13:50

Mathematics, 26.06.2019 13:50




Mathematics, 26.06.2019 13:50

Mathematics, 26.06.2019 13:50

Mathematics, 26.06.2019 13:50

Advanced Placement (AP), 26.06.2019 13:50

Advanced Placement (AP), 26.06.2019 13:50

Chemistry, 26.06.2019 13:50

English, 26.06.2019 13:50

English, 26.06.2019 13:50

Advanced Placement (AP), 26.06.2019 13:50


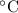 .
. specific heat capacity
specific heat capacity 

