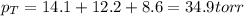Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 22.06.2019 23:30
Why do oxygen have a strong attractive force for electrons
Answers: 2

Chemistry, 23.06.2019 00:40
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1
You know the right answer?
Aflask contains argon, helium, and fluorine gases. the partial pressures of each are 14.1 torr, 12.2...
Questions


Physics, 24.03.2021 20:40

Mathematics, 24.03.2021 20:40

Health, 24.03.2021 20:40


Mathematics, 24.03.2021 20:40

Spanish, 24.03.2021 20:40





History, 24.03.2021 20:40


Mathematics, 24.03.2021 20:40


Spanish, 24.03.2021 20:40

Mathematics, 24.03.2021 20:40

Mathematics, 24.03.2021 20:40

English, 24.03.2021 20:40

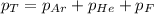
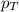 = total pressure in the flask = ?
= total pressure in the flask = ?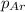 = partial pressure of argon = 14.1 torr
= partial pressure of argon = 14.1 torr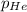 = partial pressure of helium = 12.2 torr
= partial pressure of helium = 12.2 torr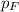 = partial pressure of fluorine = 8.6 torr
= partial pressure of fluorine = 8.6 torr