Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2

Chemistry, 23.06.2019 01:30
Which statement justifies that hydrogen peroxide (h2o2) is a polar molecule? the o – h bond is nonpolar and the molecule is asymmetric. the o – h bond is nonpolar and the molecule is symmetric. the o – h bond is polar and the molecule is asymmetric. the o – h bond is polar and the molecule is symmetric.
Answers: 1

Chemistry, 23.06.2019 04:20
Malia was able to make a paper clip float on the surface of water what will most likely happen to the paper clip if a drop of dishwashing detergent is added near it
Answers: 1
You know the right answer?
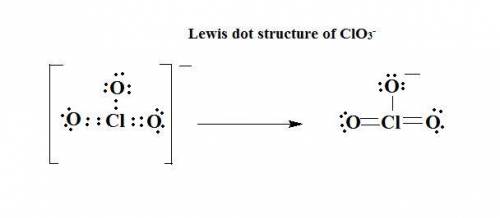
The lewis structure for a chlorate ion, clo3-, should show single bond(s), double bond(s), and lo...
Questions

Mathematics, 16.08.2021 20:10



Advanced Placement (AP), 16.08.2021 20:10


Mathematics, 16.08.2021 20:10


Mathematics, 16.08.2021 20:10


English, 16.08.2021 20:10


Mathematics, 16.08.2021 20:10

Social Studies, 16.08.2021 20:10

Medicine, 16.08.2021 20:10


Physics, 16.08.2021 20:10

Computers and Technology, 16.08.2021 20:10

Mathematics, 16.08.2021 20:10


Biology, 16.08.2021 20:10

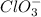 has 1 single bond, 2 double bonds and 8 lone pairs.
has 1 single bond, 2 double bonds and 8 lone pairs.