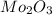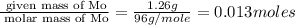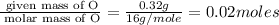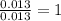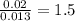Acompound that is composed of molybdenum (mo) and oxygen (o) was produced in a lab by heating molybdenum over a bunsen burner. the following data was collected:
mass of crucible: 38.26 g
mass of crucible and molybdenum: 39.52 g
mass of crucible and molybdenum oxide: 39.84 g
solve for the empirical formula of the compound, showing your calculations.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1

Chemistry, 23.06.2019 00:00
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1
You know the right answer?
Acompound that is composed of molybdenum (mo) and oxygen (o) was produced in a lab by heating molybd...
Questions




Advanced Placement (AP), 26.10.2020 07:30


English, 26.10.2020 07:30

Mathematics, 26.10.2020 07:30



Mathematics, 26.10.2020 07:30


Mathematics, 26.10.2020 07:30

Mathematics, 26.10.2020 07:30


Chemistry, 26.10.2020 07:30


Social Studies, 26.10.2020 07:30


Mathematics, 26.10.2020 07:30