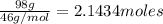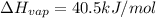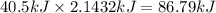Chemistry, 02.10.2019 10:50 moniquejg1800
How much energy is required to vaporize 98.6 g of ethanol (c2h5oh) at its boiling point, if its δhvap is 40.5 kj/mol? how much energy is required to vaporize 98.6 g of ethanol (c2h5oh) at its boiling point, if its δhvap is 40.5 kj/mol? 52.8 kj 11.5 kj 86.7 kj 39.9 kj 18.9 kj

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3
You know the right answer?
How much energy is required to vaporize 98.6 g of ethanol (c2h5oh) at its boiling point, if its δhva...
Questions

Mathematics, 14.11.2021 23:50




Mathematics, 14.11.2021 23:50


Mathematics, 14.11.2021 23:50


History, 14.11.2021 23:50

Chemistry, 14.11.2021 23:50




Mathematics, 14.11.2021 23:50


Mathematics, 14.11.2021 23:50

Chemistry, 14.11.2021 23:50

Mathematics, 14.11.2021 23:50


Mathematics, 14.11.2021 23:50