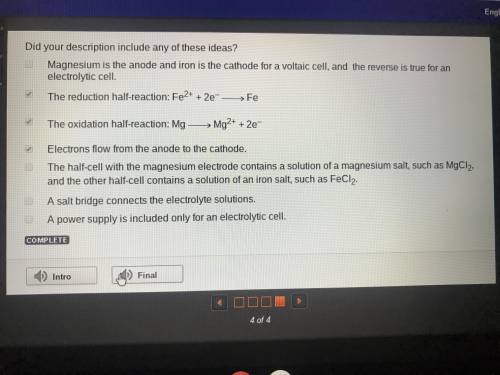
Describe an electrochemical cell based on this equation:
fe2+ + mg → mg2+ + fe
describe...

Chemistry, 19.09.2019 10:30 leannamat2106
Describe an electrochemical cell based on this equation:
fe2+ + mg → mg2+ + fe
describe all the parts and the direction of electron flow. identify the cell as either voltaic or electrolytic. write the two balanced half‐reactions.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1

Chemistry, 23.06.2019 00:00
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3

Chemistry, 23.06.2019 01:00
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2
You know the right answer?
Questions

History, 05.10.2021 14:00


English, 05.10.2021 14:00


Social Studies, 05.10.2021 14:00







Physics, 05.10.2021 14:00



Mathematics, 05.10.2021 14:00


Business, 05.10.2021 14:00

Mathematics, 05.10.2021 14:00

Social Studies, 05.10.2021 14:00

Social Studies, 05.10.2021 14:00