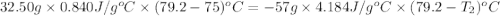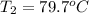Chemistry, 16.11.2019 02:31 JesusisLord2881
Apiece of glass with a mass of 32.50 g specific heat of 0.840 j/g*°c and an initial temperature of 75 °c was dropped into a calorimeter containing 57 g of water (specific heat 4.184 j/g*°c). the final temperature of the glass and water in the calorimeter was 79.2 °c. what was the initial temperature of the water?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:10
Atank contains 240 liters of fluid in which 10 grams of salt is dissolved. brine containing 1 gram of salt per liter is then pumped into the tank at a rate of 6 l/min; the well-mixed solution is pumped out at the same rate. find the number a(t) of grams of salt in the tank at time t.
Answers: 3


Chemistry, 22.06.2019 23:50
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3

Chemistry, 23.06.2019 01:00
Atoms contain subatomic particles called protons and neutrons. when these protons and neutrons spilt, a lot of energy is released
Answers: 3
You know the right answer?
Apiece of glass with a mass of 32.50 g specific heat of 0.840 j/g*°c and an initial temperature of 7...
Questions


Physics, 27.08.2019 13:20



Social Studies, 27.08.2019 13:20

Mathematics, 27.08.2019 13:20

Physics, 27.08.2019 13:20

Mathematics, 27.08.2019 13:20

Computers and Technology, 27.08.2019 13:20

History, 27.08.2019 13:20




Mathematics, 27.08.2019 13:20


Arts, 27.08.2019 13:20




Chemistry, 27.08.2019 13:20

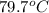
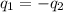
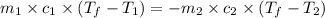
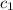 = specific heat of glass =
= specific heat of glass = 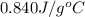
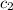 = specific heat of water =
= specific heat of water = 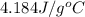
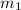 = mass of glass = 32.50 g
= mass of glass = 32.50 g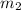 = mass of water = 57 g
= mass of water = 57 g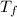 = final temperature of mixture =
= final temperature of mixture = 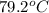
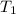 = initial temperature of glass =
= initial temperature of glass = 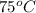
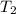 = initial temperature of water = ?
= initial temperature of water = ?