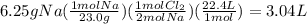Chemistry, 30.08.2019 20:30 ddmoorehouseov75lc
2. balance the equation below, and answer the following question: what volume of chlorine gas, measured at stp, is needed to complete the reaction with 6.25 g of sodium metal. (s) + ₂(g) → (s)
need !

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1

Chemistry, 23.06.2019 02:20
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2

Chemistry, 23.06.2019 04:31
2ki + pb(no3)2 → 2kno3 + pbi2 determine how many moles of kno3 are created if 0.03 moles of ki are completely consumed.
Answers: 1

You know the right answer?
2. balance the equation below, and answer the following question: what volume of chlorine gas, meas...
Questions






Social Studies, 09.01.2020 05:31

History, 09.01.2020 05:31

Advanced Placement (AP), 09.01.2020 05:31



Mathematics, 09.01.2020 05:31








Mathematics, 09.01.2020 05:31

Mathematics, 09.01.2020 05:31