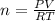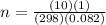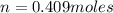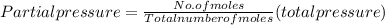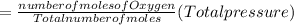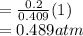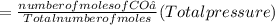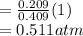Chemistry, 13.10.2019 23:30 jasminelynn135owmyj1
A10 liter flask at 298 k contains a gaseous mixture of o2 and co2 at 1 atmosphere. which statement is true for the partial pressures of o2 and co2 if 0.2 mole of o2 is present in the flask? (given the universal gas constant r = 0.082 l∙atm/k∙mol)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Does the temperature affect the solubility of sugar and salt in water? if it does tell me like different temperatures with different solubilities so i can sketch down a graph
Answers: 2


Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 12:40
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2
You know the right answer?
A10 liter flask at 298 k contains a gaseous mixture of o2 and co2 at 1 atmosphere. which statement i...
Questions

Social Studies, 01.08.2019 12:00

Mathematics, 01.08.2019 12:00


History, 01.08.2019 12:00


Physics, 01.08.2019 12:00



Business, 01.08.2019 12:00





Social Studies, 01.08.2019 12:00



History, 01.08.2019 12:00

Geography, 01.08.2019 12:00


Geography, 01.08.2019 12:00