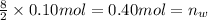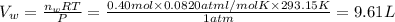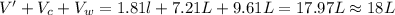Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2


Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
You know the right answer?
In an experiment, 12.0dm3 of oxygen, measured under room conditions, is used to burn completely 0.10...
Questions

History, 26.03.2021 18:10

Mathematics, 26.03.2021 18:10


Mathematics, 26.03.2021 18:10

Business, 26.03.2021 18:10

Biology, 26.03.2021 18:10


Mathematics, 26.03.2021 18:10

Spanish, 26.03.2021 18:10



Mathematics, 26.03.2021 18:10


History, 26.03.2021 18:10


Mathematics, 26.03.2021 18:10

Chemistry, 26.03.2021 18:10

Mathematics, 26.03.2021 18:10


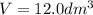 = 12.0 L
= 12.0 L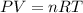
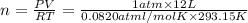
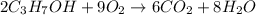
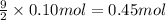
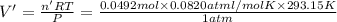
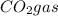
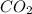 .
.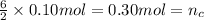
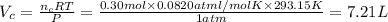
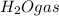
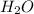 .
.