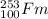Chemistry, 06.11.2019 19:31 angellinelittle
How many neutrons does the following nuclide have? 253/100 fm a. 253 b. 100 c. 153 d. none of these

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:20
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 01:50
Ase your answer to this question on the information below.hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant.the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen.chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product.nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1

Chemistry, 23.06.2019 01:00
If i had 2 m naoh solution, what does the 2 m stand for? 2 molar, but 2 of a solute in 1
Answers: 1

You know the right answer?
How many neutrons does the following nuclide have? 253/100 fm a. 253 b. 100 c. 153 d. none of these...
Questions

Mathematics, 16.12.2021 21:10

Mathematics, 16.12.2021 21:10

Physics, 16.12.2021 21:10


Mathematics, 16.12.2021 21:10


Mathematics, 16.12.2021 21:10





Mathematics, 16.12.2021 21:10

Health, 16.12.2021 21:10



Business, 16.12.2021 21:20


Business, 16.12.2021 21:20