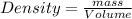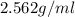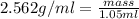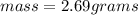Chemistry, 20.09.2019 15:30 alejandrosaaved1
What is the mass of a solid that has a density of 2.562 g/ml and displaces 1.05 ml of water?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
The most efficient way to establish the best possible economizer position is to measure
Answers: 1

Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1
You know the right answer?
What is the mass of a solid that has a density of 2.562 g/ml and displaces 1.05 ml of water?...
Questions

Mathematics, 26.09.2019 03:30

Mathematics, 26.09.2019 03:30









Biology, 26.09.2019 03:30

Mathematics, 26.09.2019 03:30

Biology, 26.09.2019 03:30

Mathematics, 26.09.2019 03:30

Mathematics, 26.09.2019 03:30




English, 26.09.2019 03:30