
Chemistry, 03.10.2019 08:00 student679
C-12 and c-13 are naturally occurring isotopes of the element carbon. c-12 occurs 98.89% of the time and c-13 occurs 1.108% of the time. what calculation should be used to determine the atomic mass of this element?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1

Chemistry, 22.06.2019 04:00
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2
You know the right answer?
C-12 and c-13 are naturally occurring isotopes of the element carbon. c-12 occurs 98.89% of the time...
Questions












Mathematics, 06.05.2020 15:59

Mathematics, 06.05.2020 15:59

Mathematics, 06.05.2020 15:59

Social Studies, 06.05.2020 15:59





Computers and Technology, 06.05.2020 15:59

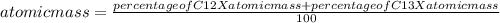
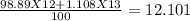 g/mol
g/mol

