

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1

You know the right answer?
Bonding atoms that experience large differences in electronegativity values will experience what typ...
Questions


Chemistry, 14.12.2020 18:40

French, 14.12.2020 18:40


Chemistry, 14.12.2020 18:40


Mathematics, 14.12.2020 18:40




Mathematics, 14.12.2020 18:40

Mathematics, 14.12.2020 18:40


Mathematics, 14.12.2020 18:40

Mathematics, 14.12.2020 18:40

Mathematics, 14.12.2020 18:40

Mathematics, 14.12.2020 18:40

Mathematics, 14.12.2020 18:40

Mathematics, 14.12.2020 18:40

Mathematics, 14.12.2020 18:40

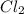 molecule has covalent bond.
molecule has covalent bond.

