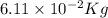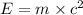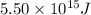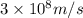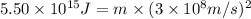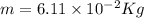The mass defect of a particular reaction resulted in the production of 5.50 x 10^15 joules of energy. what mass (if totally converted to energy) would correspond to this amount of energy? (1 j = 1 kg m^2/s^2)
1.83 x 10^8 kg
4.95 x 10^7 kg
6.11 x 10^-2 kg
1.64 x 10^-1 kg

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Consider the point on the plot where 10.0 g of naoh have been added. what amount of naoh, in moles, has been added? 0.308 mol fecl3 initially present
Answers: 1

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 04:30
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2
You know the right answer?
The mass defect of a particular reaction resulted in the production of 5.50 x 10^15 joules of energy...
Questions


Mathematics, 25.02.2021 21:50


Mathematics, 25.02.2021 21:50







Chemistry, 25.02.2021 21:50


Physics, 25.02.2021 21:50


Physics, 25.02.2021 21:50

Mathematics, 25.02.2021 21:50

History, 25.02.2021 21:50

English, 25.02.2021 21:50

Physics, 25.02.2021 21:50