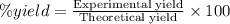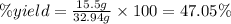Chemistry, 30.09.2019 12:00 Juliette9525
One method to produce nitrogen in the lab is to react ammonia with copper (ii) oxide: nh3(
g. + cuo(s) cu(s) + h2o(l) + n2(
g. after using 40.0 grams of nh3, 15.5 grams of n2 are produced. what is the percent yield of nitrogen in the reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 23.06.2019 00:00
How many atoms or molecules are there in a mole of a substance?
Answers: 1
You know the right answer?
One method to produce nitrogen in the lab is to react ammonia with copper (ii) oxide: nh3(
g....
g....
Questions




Mathematics, 25.05.2021 01:00





Mathematics, 25.05.2021 01:00

Mathematics, 25.05.2021 01:00

Mathematics, 25.05.2021 01:00


Chemistry, 25.05.2021 01:00

Advanced Placement (AP), 25.05.2021 01:00

Mathematics, 25.05.2021 01:00

Health, 25.05.2021 01:00




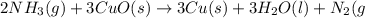
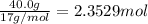
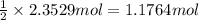 of nitrogen gas
of nitrogen gas