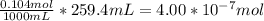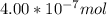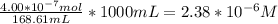Chemistry, 24.09.2019 12:00 divadebbgirl1
When a chemist titrates a standard solution of 168.61 ml of hydrochloric acid (hcl) with 0.104 m sodium hydroxide (naoh) , she finds that it requires 259.4 ml of the base to reach the endpoint of the titration. what is the molarity of the acid solution ?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3

Chemistry, 23.06.2019 03:30
Ahelium balloon contains 16.9 l of helium at stp. how many atoms of helium are in the balloon
Answers: 1
You know the right answer?
When a chemist titrates a standard solution of 168.61 ml of hydrochloric acid (hcl) with 0.104 m sod...
Questions

Mathematics, 22.11.2019 04:31

Social Studies, 22.11.2019 04:31


Mathematics, 22.11.2019 04:31


History, 22.11.2019 04:31

History, 22.11.2019 04:31


History, 22.11.2019 04:31

Business, 22.11.2019 04:31

Mathematics, 22.11.2019 04:31

Health, 22.11.2019 04:31

History, 22.11.2019 04:31


Mathematics, 22.11.2019 04:31

Social Studies, 22.11.2019 04:31




English, 22.11.2019 04:31