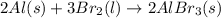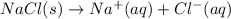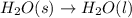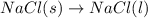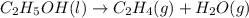Chemistry, 29.09.2019 05:30 rissacoob7862
Which of the following processes would you expect to have a negative value for entropy? nacl(s) na+(aq) + cl- (aq) h2o(s) h2o(l) nacl(s) nacl(l) 2 al(s) + 3br2(l) 2albr3(s) c2h5oh(l) c2h4(g) + h2o(g)

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 21:00
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1

Chemistry, 23.06.2019 04:10
Which of the following is described by the equation h2o(s)+ heat=h2o(i) a freezing melting condensing evaporating
Answers: 2

Chemistry, 23.06.2019 10:30
Ireally need ! calcium metal reacts with a potassium chloride solution to form calcium chloride and potassium ions. balance this reaction. (s) + (aq) → cacl2(s) + +(aq) a) 1, 2, 1, 2 b) 1, 2, 1, 1 c) 1, 1, 1, 1 d) 2, 1, 2, 1
Answers: 1
You know the right answer?
Which of the following processes would you expect to have a negative value for entropy? nacl(s) na+...
Questions

Mathematics, 30.04.2021 03:00

Spanish, 30.04.2021 03:00



Arts, 30.04.2021 03:00

History, 30.04.2021 03:00


French, 30.04.2021 03:00






Mathematics, 30.04.2021 03:00



Social Studies, 30.04.2021 03:00



Geography, 30.04.2021 03:00