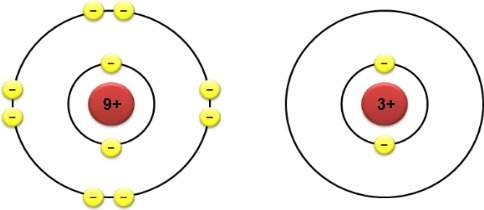
Given the following reactions and subsequent ∆h values what is the ∆h for the reaction below?
...


Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Acycloalkane molecule contains 8 carbon atoms. how many hydrogen atoms are present in the molecule?
Answers: 2

Chemistry, 21.06.2019 20:30
12. complete each of the following word equations for synthesis reactions. a. sodium + oxygen -> b. magnesium + fluorine -> 13. complete and balance the equations for the decomposition reactions. a. hgo -> [with the triangle heat symbol above the arrow] b. h2o(l) -> [with "electricity" written above the arrow]
Answers: 1

Chemistry, 22.06.2019 00:30
In numbering carbon atoms in the parent chain of a hydrocarbon, why would you number from right to left, rather than left to right
Answers: 1

Chemistry, 22.06.2019 01:30
What is the value of keq for the reaction expressed in scientific notation
Answers: 1
You know the right answer?
Questions

Mathematics, 12.02.2021 17:30



Mathematics, 12.02.2021 17:30

Mathematics, 12.02.2021 17:30



Health, 12.02.2021 17:30





Chemistry, 12.02.2021 17:30

Mathematics, 12.02.2021 17:30




Mathematics, 12.02.2021 17:30

Mathematics, 12.02.2021 17:30

Mathematics, 12.02.2021 17:30