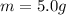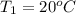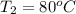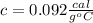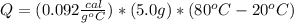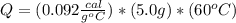Chemistry, 01.09.2019 12:30 chevalieriiim006
5.0 g of copper was heated from 20 degrees celsius to 80 degrees celsius. how much energy was used to heat cu( specific heat capacity of cu is 0.092 cal/g degrees celsius)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Asmall amount of a solid is added to water. the observation made after fifteen minutes is shown in the figure. which of these solids has been probably added to water? a) oil b) sand c) sugar d) wood chips
Answers: 1

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2


Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2
You know the right answer?
5.0 g of copper was heated from 20 degrees celsius to 80 degrees celsius. how much energy was used t...
Questions

Chemistry, 10.01.2021 01:30

Social Studies, 10.01.2021 01:30





English, 10.01.2021 01:30

Computers and Technology, 10.01.2021 01:30

English, 10.01.2021 01:30


Mathematics, 10.01.2021 01:30






Mathematics, 10.01.2021 01:30


Computers and Technology, 10.01.2021 01:30

Mathematics, 10.01.2021 01:30

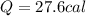
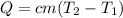
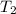 and
and